What is anodizing?
Anodizing is an electrochemical process used to form a stable oxide layer on metal surfaces. As the name suggests, the metal to be oxidized must act as the anode in the electrochemical reaction, requiring a suitable electrolyte and a cathodic material. Since the oxide layer on aluminum alloys forms through a reaction with the base metal, it prevents peeling or flaking.
Aluminum and its alloys are the most common metals for anodizing, with other suitable metals including magnesium, zinc, zirconium, titanium, and niobium. However, anodizing is not applicable to ferrous metals like carbon steel because iron oxide (rust), containing FeO and Fe2O3, is porous and loosely adheres to the substrate, unable to provide effective passivation.
The function of anodizing:
- Enhances the wear resistance of aluminum alloys. The high hardness of aluminum oxide, reaching a Mohs hardness level of 9 (second only to diamond), significantly improves the overall wear resistance of components as the thickness of the oxide layer increases.
- Increases the corrosion resistance of aluminum alloys. The natural oxide layer on the surface of aluminum alloys is approximately 25 – 30μm thick and provides good passivation in normal atmospheric conditions. However, under more severe conditions, it may not adequately protect the aluminum substrate. A thicker oxide layer better isolates the base metal, enhancing resistance to pitting corrosion.
- The transparent nature of the aluminum oxide layer enhances the metal’s appearance. Thicker oxide layers improve aesthetics, and parts with porous oxide layers can be further enhanced through coloring.
- Anodized components exhibit good insulation properties.
The mechanism of anodizing:
Aluminum alloy anodizing is a surface passivation treatment technique performed through an electrolytic reaction in an electrolyte bath. The specific process involves placing aluminum or aluminum alloy as the anode in a suitable electrolyte and connecting it to the positive terminal of an external power source. Simultaneously, the cathode (which can be made of materials such as platinum, lead, stainless steel, or carbon rods) is immersed in the electrolyte and connected to the negative terminal of the power source. When the circuit is completed, the aluminum alloy, acting as the anode in the electrolytic cell, loses electrons and undergoes oxidation, resulting in the formation of an oxide film on the surface.
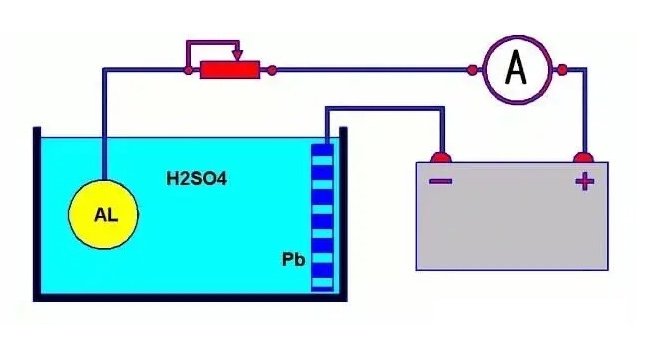
The external power source can be direct current, alternating current, or a combination of both. The choice of electrolyte is relatively flexible. Strongly acidic electrolytes such as sulfuric acid or phosphoric acid are typically selected for the preparation of porous oxide films. Other types of electrolytes generally form non-porous “barrier” type oxide films, such as ammonium tartrate, ammonium borate, etc. Sulfuric acid solution is the most widely used electrolyte in industrial anodizing processes.
According to the US military standard MIL-A-8625, the anodizing process can be classified into the following three types:
I: Using chromic acid as the electrolyte, resulting in a relatively thin oxide layer;
II: Using sulfuric acid as the electrolyte, producing a thicker and more porous oxide layer, suitable for coloring;
III: Similar to Type II process, but requiring the formation of a thicker oxide layer, also known as hard anodizing.
Based on whether dyeing is performed, the three types of anodizing processes can be categorized into two categories: Category 1 (uncolored anodizing) and Category 2 (colored anodizing).
Types of Oxide Films:
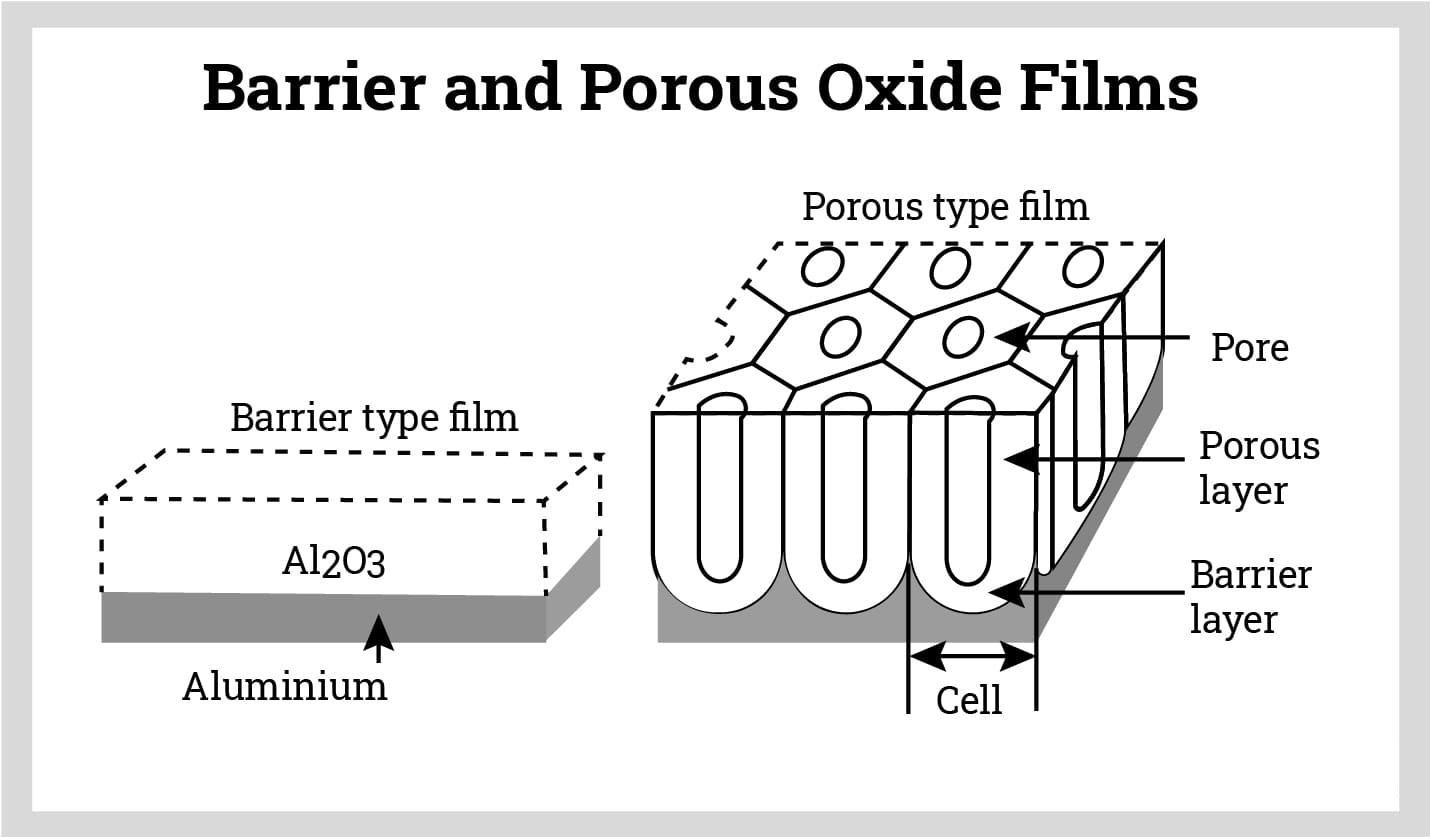
Anodized oxide films can be classified into barrier-type and porous-type based on the presence of pores.
Barrier-type: Oxide films obtained using neutral electrolytes (e.g., ammonium borate, phosphates) are typically robust and dense. These films provide strong protection to the substrate. The thickness of barrier-type oxide films depends on the voltage applied between the cathode and anode.
Porous-type: Using acidic electrolytes (e.g., 10% sulfuric acid) typically results in porous oxide films. Initially, the oxide film is quite dense, but with continuous current flow, the more reactive portions of the oxide film gradually detach due to the continuous action of the current and dissolve into the acidic electrolyte. Eventually, a porous oxide film is formed. The thickness of this type of film is proportional to the anodizing time and voltage, while the pore size depends on the voltage, electrolyte temperature, and acid concentration.

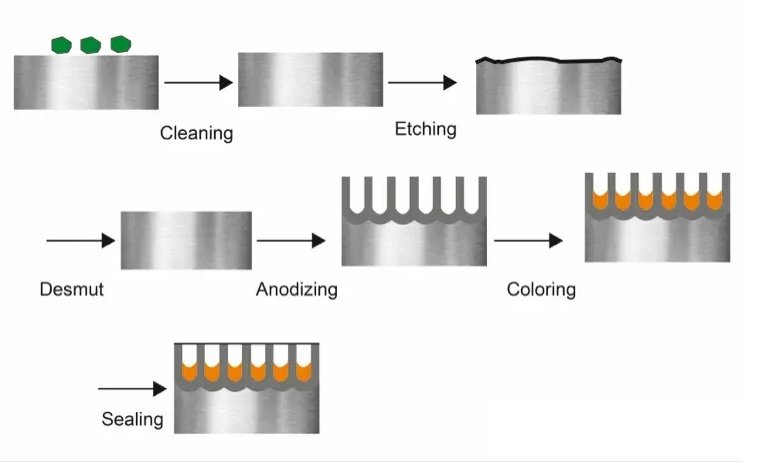
Steps of Anodizing
- Pre-treatment
Pre-treatment mainly involves cleaning and chemical treatment of the workpiece surface. Grease, oil, and other impurities may cause defects or color differences on the finished anodized surface, so the surface must be thoroughly cleaned. If necessary, the surface can be chemically etched to ensure that it is free from defects.
- Anodizing
The primary consideration for selecting the anodizing process is the chemical composition and microstructure of the aluminum alloy. Additionally, the metallurgical processing history of the workpiece (such as casting, forging, heat treatment status, and the presence of welds) is also an important consideration. The process parameters for anodizing include electrolyte temperature, current density, voltage, and anodizing time, among others.
- Coloring
After anodizing treatment in acidic electrolyte, the workpiece surface forms a porous oxide layer. This porous structure can absorb other materials like a sponge, thereby changing the surface state of the workpiece. Since the oxide layer itself is colorless, the surface of the workpiece presents different colors after adsorbing dyes or pigments.

- Sealing
Sealing is the final step of the anodizing process, which aims to close the pores of the oxide layer from the outside to ensure that the workpiece surface is in a more stable state. After sealing, anodized workpieces are enhanced in terms of corrosion resistance, wear resistance, and color stability.
Conclusion
Anodizing treatment can significantly improve the durability and aesthetics of aluminum alloy parts. It is an important process technology in the aluminum processing industry and is widely used in industries such as defense, aerospace, automotive manufacturing, consumer electronics, furniture, and household appliances. Although the anodizing process brings about an increase in production costs and complexity, it plays a positive role in ensuring that we obtain higher-quality products.